Introduction
The global healthcare landscape is undergoing a profound transformation, driven by the emergence and rapid advancement of advanced therapeutics. These therapies, which include gene therapy, cell therapy, immunotherapy, and tissue-engineered products, are redefining how we treat and potentially cure complex diseases. Unlike conventional treatments that manage symptoms, advanced therapeutics aim to modify the underlying biology of disease, offering the possibility of long-term or permanent remission.
This article explores the current state of the advanced therapeutics market, examining key trends, driving forces, challenges, and the market’s outlook over the coming decade.
What Are Advanced Therapeutics?
Advanced therapeutics represent a frontier in biomedical innovation. These treatments fall into several categories:
-
Gene Therapy: Involves modifying or replacing defective genes to treat or prevent disease. It holds promise for genetic disorders like spinal muscular atrophy (SMA) and certain cancers.
-
Cell Therapy: Uses living cells to repair or replace damaged tissues. CAR T-cell therapies, which modify a patient’s immune cells to attack cancer, are a key example.
-
Tissue Engineering: Combines cells, engineering methods, and materials to develop biological substitutes for damaged organs or tissues.
-
Immunotherapy: Enhances the body’s natural defenses to fight diseases, especially cancers and autoimmune conditions.
These therapies are not only groundbreaking in their approach but also require advanced manufacturing, distribution, and administration models, which are rapidly evolving in response to demand.
Market Overview
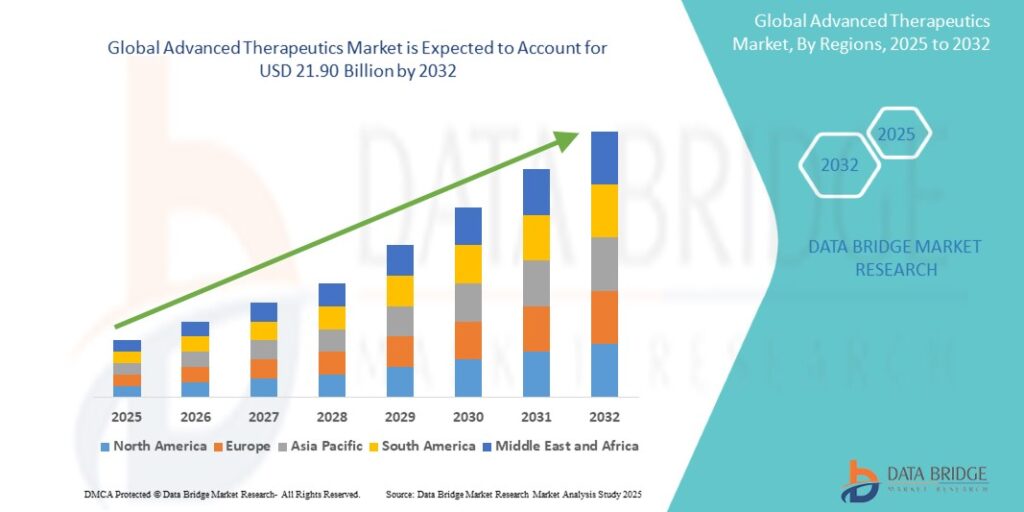
The global advanced therapeutics market was valued at approximately USD 50 billion in 2023 and is projected to surpass USD 150 billion by 2030, growing at a CAGR of over 15%. This growth is driven by increasing approvals, rising investment, and greater demand for personalized medicine.
Key market segments include:
-
Oncology: The largest share, particularly through CAR T-cell therapies and immune checkpoint inhibitors.
-
Genetic Disorders: Conditions like hemophilia, Duchenne muscular dystrophy, and SMA are seeing breakthroughs.
-
Autoimmune Diseases: Therapies targeting conditions like lupus, Crohn’s disease, and rheumatoid arthritis.
-
Rare Diseases: Due to orphan drug incentives, biotech firms are increasingly targeting ultra-rare conditions.
Drivers of Market Growth
-
Scientific Innovation and Research
Advances in genomics, CRISPR-based gene editing, and stem cell research have created an explosion in therapeutic potential. Academic institutions and biotech firms are at the forefront, often supported by venture capital and government grants. -
Regulatory Support
Agencies such as the U.S. FDA and EMA have introduced fast-track, breakthrough, and orphan designations to speed up development and approval. The FDA’s approval of gene therapies like Zolgensma and Luxturna marks a shift toward regulatory acceptance of novel modalities. -
Rising Prevalence of Chronic and Genetic Diseases
As the global burden of non-communicable diseases grows, the need for long-lasting or curative treatments becomes more urgent. Genetic and degenerative diseases, once deemed untreatable, are now at the center of therapeutic innovation. -
Increasing Investments and Partnerships
Pharmaceutical giants are investing heavily in biotech start-ups, forming strategic alliances to bring advanced therapeutics to market. Deals often exceed billions of dollars, underscoring the confidence in these emerging modalities.
Challenges in the Advanced Therapeutics Market
Despite the promising growth, the market faces several barriers:
-
High Costs
Gene and cell therapies can cost upwards of USD 1 million per treatment, raising concerns about affordability and access. This has spurred discussions on new reimbursement models, including outcomes-based pricing. -
Manufacturing Complexities
Producing living therapies requires specialized facilities, rigorous quality controls, and cold chain logistics. Scaling up from clinical to commercial production is a significant hurdle. -
Regulatory and Ethical Concerns
Ethical debates, especially around germline gene editing, and evolving regulatory standards pose uncertainties for developers. Ensuring safety and long-term monitoring is crucial. -
Limited Healthcare Infrastructure
In many parts of the world, healthcare systems are not yet equipped to handle the administration of these complex therapies, particularly in rural or resource-limited settings.
Regional Market Insights
-
North America: Leads the market with robust R&D infrastructure, early regulatory approvals, and strong investment activity. The U.S. dominates with companies like Novartis, Gilead Sciences, and Spark Therapeutics.
-
Europe: Strong research environment and supportive policies, particularly in the UK, Germany, and France. EMA has facilitated several advanced therapy approvals.
-
Asia-Pacific: Fast-growing market due to government support, large patient populations, and rising biotech activity. China and Japan are key players, with increasing clinical trials and approvals.
-
Latin America and Middle East: Emerging markets with increasing interest, though access and infrastructure remain limiting factors.
Competitive Landscape
The advanced therapeutics market is highly competitive, involving a mix of established pharmaceutical companies and innovative biotechnology firms. Key players include:
-
Novartis: A pioneer in CAR T-cell therapies with Kymriah.
-
Gilead Sciences/Kite Pharma: Active in cell therapy development.
-
Bluebird Bio: Focuses on gene therapies for rare diseases.
-
Pfizer and Roche: Investing heavily in gene therapy pipelines.
-
CRISPR Therapeutics, Editas Medicine, Intellia Therapeutics: Leading gene editing firms exploring next-generation therapies.
Collaborations, licensing deals, and mergers are common as companies race to expand pipelines and access proprietary technologies.
Future Outlook
The advanced therapeutics market is poised for transformative growth. Key trends to watch include:
-
Gene Editing Evolution: The emergence of next-generation gene editing technologies like base and prime editing could offer more precise and safer therapies.
-
Off-the-Shelf Cell Therapies: Allogeneic therapies (from donors rather than the patient) could simplify logistics and reduce costs.
-
AI and Big Data Integration: Leveraging artificial intelligence in drug discovery and patient selection can accelerate development and enhance success rates.
-
Global Expansion: Efforts to expand access and manufacturing capacity in emerging markets will help democratize advanced therapeutics.
Conclusion
Advanced therapeutics represent a paradigm shift in medicine, offering hope for previously untreatable diseases and the potential for long-term cures. While the market faces challenges related to cost, scalability, and access, the momentum of scientific innovation, investment, and regulatory evolution suggests a bright future.
As stakeholders continue to align on making these therapies more accessible and sustainable, advanced therapeutics will increasingly become a cornerstone of modern medicine, fundamentally altering the healthcare landscape for years to come.
Get More Details
https://www.databridgemarketresearch.com/reports/global-advanced-therapeutics-market